Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kagami, Saya; Yokoyama, Tetsuya*
Geostandards and Geoanalytical Research, 45(4), p.679 - 699, 2021/12
Times Cited Count:2 Percentile:21.91(Geochemistry & Geophysics)We developed a new method for the determination of the mass fractions of insoluble fluoride-forming elements (IFFEs) and high field strength elements (HFSEs) in rock samples by ICP-QMS. Unlike conventional methods in which the two elemental groups were measured separately using two solutions prepared from distinct sample aliquots, the new technique prepares a solution by acid digestion from a single sample aliquot, then divide the solution into two fractions that are dedicated to the measurement of IFFEs and HFSEs, respectively. The problem regarding the incorporation of IFFEs/HFSEs into insoluble fluorides during HF digestion was overcome by adjusting the Ca-Al-Mg composition of the sample before acid digestion. The acceptable compositional range was Ca/(Ca+Al)  0.43 and Mg/(Mg+Al)
0.43 and Mg/(Mg+Al)  0.40 when the sample was decomposed on a hot plate, while more restricted condition of Ca/(Ca+Al)
0.40 when the sample was decomposed on a hot plate, while more restricted condition of Ca/(Ca+Al)  0.40, Mg/(Mg+Al)
0.40, Mg/(Mg+Al)  0.40, and Al/(Mg+Ca)
0.40, and Al/(Mg+Ca)  1.7 was required for the digestion under high pressure and temperature using Teflon bomb. This method achieved the repeatabilities of
1.7 was required for the digestion under high pressure and temperature using Teflon bomb. This method achieved the repeatabilities of  4% for most IFFEs and HFSEs, and yielded the mass fractions of most IFFEs and HFSEs that were consistent with the data obtained by conventional methods within
4% for most IFFEs and HFSEs, and yielded the mass fractions of most IFFEs and HFSEs that were consistent with the data obtained by conventional methods within  5%. The method is applicable not only to precious samples but also to heterogeneous samples containing accessory minerals enriched in IFFEs/HFSEs.
5%. The method is applicable not only to precious samples but also to heterogeneous samples containing accessory minerals enriched in IFFEs/HFSEs.
Miyajima, Yusuke*; Saito, Ayaka*; Kagi, Hiroyuki*; Yokoyama, Tatsunori; Takahashi, Yoshio*; Hirata, Takafumi*
Geostandards and Geoanalytical Research, 45(1), p.189 - 205, 2021/03
Times Cited Count:5 Percentile:21.77(Geochemistry & Geophysics)Uncertainty for elemental and isotopic analyses of calcite by LA-ICP-MS is largely controlled by the homogeneity of the reference materials (RMs) used for normalization and validation. In order to produce calcite RMs with homogeneous elemental and isotopic compositions, we incorporated elements including U, Pb, and rare earth elements into calcite through heat- and pressure-induced crystallization from amorphous calcium carbonate that was precipitated from element-doped reagent solution. X-ray absorption spectra showed that U was present as U(VI) in the synthesized calcite, probably with a different local structure from that of aqueous uranyl ions. The uptake rate of U by our calcite was higher in comparison to synthetic calcite of previous studies. Variations of element mass fractions in the calcite were better than 12% 2RSD, mostly within 7%. The 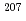 Pb/
Pb/ Pb ratio in the calcite showed
Pb ratio in the calcite showed  1% variations, while the
1% variations, while the  U/
U/ Pb ratio showed 3-24% variations depending on element mass fractions. Using the synthetic calcite as primary RMs, we could date a natural calcite RM, WC-1, with analytical uncertainty as low as
Pb ratio showed 3-24% variations depending on element mass fractions. Using the synthetic calcite as primary RMs, we could date a natural calcite RM, WC-1, with analytical uncertainty as low as  3%. The method presented can be useful to produce calcite with controlled and homogeneous element mass fractions, and is a promising alternative to natural calcite RMs for U-Pb geochronology.
3%. The method presented can be useful to produce calcite with controlled and homogeneous element mass fractions, and is a promising alternative to natural calcite RMs for U-Pb geochronology.